Relationship between relative humidity difference and moisture permeability in moisture permeability test
Abstract: water vapor molecules are polar molecules, while common inorganic gas molecules are nonpolar molecules. Due to the strong interaction between water vapor molecules and hydrophilic polymers, the permeation mechanism of water vapor to polymers is different from that of common inorganic gases. This paper discusses in detail the relationship between the moisture permeability and the relative humidity difference of different polymers in the moisture permeability test, and introduces the influence of humidity change on the permeability of common inorganic gases.
Key words: moisture permeability, relative humidity difference, molecular polarity, polar polymer, hydrophilic polymer, hydrophobic polymer
The existence of water vapor can cause serious qualitative changes in food, drugs and electronic products. Therefore, the moisture permeability test of packaging materials has attracted extensive attention of all relevant industries, and the detection level and detection equipment have developed very rapidly.
The permeation mechanism of water vapor to polymer is similar to that of common inorganic gas to polymer, but water vapor is polar molecule, while common inorganic gas is non-polar molecule. The difference of molecular properties directly affects the specific permeation process and permeation amount.
1. Moisture permeability test and common influencing factors
Because water vapor molecules are polar molecules, the permeation process does not fully comply with Fick's law, and the specific permeation situation varies according to different materials, so it is still difficult to summarize a unified formula at present. When the solubility of water vapor molecules in the polymer is not large, the calculation of permeability Q approximately meets Fick's law. The moisture permeability test standard adopts Fick type diffusion model to simulate and calculate the moisture permeability (water vapor permeability) and moisture permeability coefficient (water vapor permeability coefficient) of the material. When the temperature is constant, the transmission area is constant, and the relative humidity difference (water vapor differential pressure) on both sides of the sample is constant, the moisture permeability within a certain time can be directly measured by the cup method (weighing method), and the test can be calculated
The moisture permeability coefficient of the sample is as follows:
Δm——t Mass increment in time,g;
A——Area of pervious steam of the sample,m2;
d——Specimen thickness,cm;
Δp——Relative humidity difference on both sides of the sample (water vapor differential pressure), Pa。
As with the permeability test of materials, the water vapor permeability of materials is related to their crystallinity, density, and tensile orientation. Temperature is one of the main factors affecting the barrier test. Generally, the moisture permeability of materials increases significantly with the increase of temperature. Because there is basically no interaction between common inorganic gases and polymer materials, and the gas molecules dissolved into the polymer will not be fixedly adsorbed in the polymer, but water vapor is a polar molecule. In the process of water vapor penetration into polar polymers, some polymers will first absorb water vapor and swell, which will increase the free volume. The moisture permeability coefficient of materials is obviously dependent on the concentration of water vapor, Accordingly, the moisture permeability of materials is also affected by humidity changes.
2 、Influence of relative humidity difference on test results
Languang laboratory has conducted a large number of moisture permeability tests for the same film under different relative humidity differences in the past three months. The test film is 25 μ M pure PET film, and the test equipment is Labthink tsy-t1 moisture permeability tester. Some test results are listed in Table 1.
Table 1. Measured data of 38 ℃ pet under different relative humidity differences
Humidity difference control% RH | number | Experimental humidity difference% RH | Test temperature℃ | experimental resultg/㎡•24h | S | CV%0 |
| 28 | 1 | 27.6 | 38.1 | 2.45 |
|
|
| 2 | 27.6 | 37.9 | 2.64 |
|
|
| 3 | 28.2 | 38 | 2.40 |
|
|
| 4 | 28.5 | 38 | 2.17 |
|
|
| 5 | 28.7 | 37.9 | 2.87 |
|
|
| Mean | 28.1 | 38 | 2.51 | 0.26 | 10.50 |
30
| 1 | 29.4 | 38 | 3.11 |
|
|
| 2 | 29.4 | 38 | 3.01 |
|
|
| 3 | 30.2 | 38 | 3.96 |
|
|
Mean
| 29.7 | 38 | 3.36 | 0.52 | 15.54 |
85
| 1 | 84.7 | 38 | 10.94 |
|
|
| 2 | 84.9 | 38 | 10.56 |
|
|
| 3 | 85 | 38 | 10.75 |
|
|
| 4 | 85.5 | 38 | 11.32 |
|
|
| 5 | 85.6 | 38 | 10.75 |
|
|
| Mean | 85.1 | 38 | 10.86 | 0.29 | 2.65 |
90
| 1 | 89.8 | 38 | 11.32 |
|
|
| 2 | 89.9 | 38 | 11.70 |
|
|
| 3 | 89.9 | 38 | 11.51 |
|
|
| 4 | 90.4 | 38 | 11.70 |
|
|
| 5 | 90.6 | 38 | 11.32 |
|
|
| Mean | 90.1 | 38 | 11.51 | 0.19 | 1.65 |
95
| 1 | 94.3 | 38 | 11.88 |
|
|
| 2 | 94.9 | 38 | 11.70 |
|
|
| 3 | 94.9 | 38 | 11.70 |
|
|
| 4 | 94.9 | 38 | 11.70 |
|
|
| 5 | 94.9 | 38 | 12.70 |
|
|
| Mean | 94.8 | 38 | 11.81 | 0.16 | 1.40 |
97
| 1 | 96.4 | 38 | 12.07 |
|
|
| 2 | 96.5 | 38 | 12.07 |
|
|
| 3 | 96.6 | 38 | 12.26 |
|
|
| 4 | 96.6 | 38 | 12.45 |
|
|
| 5 | 96.7 | 38 | 13.02 |
|
|
| Mean | 93.6 | 38 | 12.37 | 0.16 | 1.26 |
It can be seen from the data in the table that the range of relative humidity difference for the test is narrow, and there are two main reasons: first, if you want to control a stable 30% RH ~ 80% RH humidity environment, the most effective way is to use saturated salt solution, but its preparation process is relatively complex, requires a large number of chemical products, and the test efficiency is low; Secondly, because the tsy-t1 moisture permeability tester used in the test adopts the measurement principle of weight reduction method, the interior of its moisture permeability cup is a 100% RH wet environment. If the relative humidity difference on both sides of the sample is to be controlled within 30%, it is necessary to provide very good air circulation conditions in the test environment.
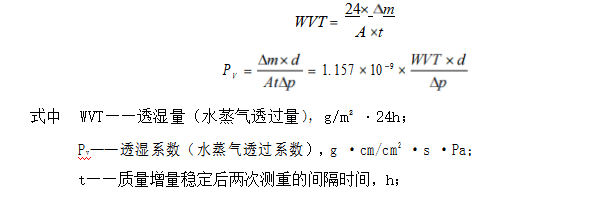
The calculated data in Table 1 are plotted as follows:
In Figure 1, the moisture permeability of pet is basically linear with the relative humidity difference on both sides of the sample, but it fluctuates greatly. Since no test data are obtained in the range of relative humidity difference of 30% RH ~ 80% RH, it is impossible to draw a conclusion whether the moisture permeability of PET materials is approximately in line with the relative humidity difference in the range of 0% RH ~ 100% RH.
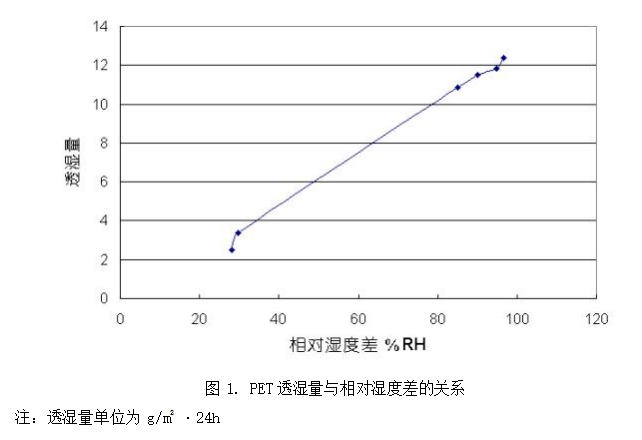
The linear relationship between moisture permeability and relative humidity difference is not valid for all polymers. As shown in Figure 2 (extracted from plastic testing technology), the relationship between moisture permeability and relative humidity difference of hydrophilic polymer cellulol is not linear. With the increase of relative humidity difference, the increase of moisture permeability increases significantly. This phenomenon that the permeability is not linearly related to the differential pressure (for moisture permeability test, it is the relative humidity difference) is the most significant difference between water vapor and common inorganic gases in the process of penetrating through polymers.
3. Reasons why the change of relative humidity difference affects the test results
The relative humidity difference has some influence on the test results, but not for all polymers. Here, we will discuss the characteristics of permeable gas molecules and polymers.
3.1 permeable gas
3.1.1 inorganic gas molecules
The common inorganic gas molecules have no polarity, and there is basically no interaction with the polymer. The polymer structure is rarely affected by the permeable gas. The air permeability obtained from the test is linearly proportional to the pressure difference of the test gas. Therefore, the data under other pressure differences can be easily calculated from the data obtained from the test under one pressure difference. Just as when we carry out the isobaric oxygen permeability test of materials, if the oxygen permeability of materials is too large, we usually use the method of reducing the oxygen concentration difference on both sides of the sample to expand the detection range of the equipment. For example, replace pure oxygen with air (oxygen content 20.8%), and only increase the test result obtained by the sample under the oxygen concentration difference of 20.8% by 4.8 times to obtain the oxygen permeability and oxygen transmittance of the sample under the oxygen concentration difference of 100%. For the permeability test of differential pressure method, the differential pressure difference of the test gas on both sides of the sample can be controlled by increasing or decreasing the test gas pressure on the high-pressure side. The permeability of the material is directly proportional to the pressure difference of the test gas, and the gas permeability (permeability coefficient) is not affected.
3.1.2 water vapor molecules
The difference between water vapor and common inorganic gases when penetrating through polymers is mainly related to the difficulty of condensation and the polarity of water vapor molecules.
Compared with common inorganic gases, water vapor that is easy to condense is easier to dissolve in polymers. The high polarity of water vapor molecules and their tendency to form hydrogen bonds make their transmittance very different from common inorganic gases, especially for polar polymers. Hydrogen bond is the main intermolecular force in polymer materials. Because hydrogen bond can be formed between water vapor molecules and polar polymer groups, the movement of water vapor molecules in polymers is significantly affected. The solubility coefficient of water vapor on the surface of polymer and the diffusion coefficient in polymer are affected by the interaction between water vapor and polymer. Table 2 shows the molar ratio of water absorption of each polymer structural unit under different relative humidity at 25 ℃ given in plastic packaging materials for food. It can be seen that the water absorption of polar polymers will change under the same temperature and different relative humidity according to their different structures. Especially for hydrophilic polymers, each polar OH group has a strong interaction with water molecules. The number of water molecules adsorbed on the polymer surface is different from that dissolved in polymerization according to Henry's law
The number of water molecules in the matrix can be in the same order of magnitude.
| Group | relative humidity |
| 0.3 | 0.5 | 0.7 | 0.9 | 1.0 |
-CH3 | (1.5×10-3) | (2.5×10-3) | (3.3×10-3) | (4.5×10-3) | (5×10-3) |
-CH2-CH= | 0.001
| 0.002 | 0.003 | 0.004 | 0.005 |
| =C=O | 0.025 | 0.255 | (0.11) | (0.20) | (0.3) |
| 0.025 | 0.05 | 0.075 | 0.14 | 0.2 |
| O= | 0.006 | 0.01 | 0.02 | 0.06 | 0.1 |
| -OH | 0.35 | 0.5 | 0.75 | 1.5 | 2 |
-NH2 | 0.35 | 0.5 | 0.75 | (1.5) | (2) |
| -NH3+ |
|
| 2.8 | 5.3 |
|
| -COOH | 0.2 | 0.3 | 0.6 | 1.0 | 1.3 |
| -COO- | 1.1 | 2.1 | 4.2 |
|
|
| 0.35 | 0.5 | 0.75 | 1.5 | 2 |
-C1
| 0.003
| 0.006 | 0.015 | 0.06 | (0.1) |
| -CH | 0.015 | 0.02 | 0.065 | 0.22 | (0.3) |
If the interaction between water vapor and polymer is strong, the film swells due to dissolution and adsorption, which increases the free volume. Therefore, the polymer structure affects the permeation process due to the interaction with water vapor. Generally, the adsorption and swelling of water vapor on the film will significantly increase the permeability of all gases to the film, which is related to the concentration of water vapor that can cause the film to swell. On the one hand, this can explain why for some polymers (which interact strongly with water vapor, such as EVOH, PA and other barrier materials), the water vapor transmittance is much greater than the oxygen and nitrogen transmittance of the materials; On the other hand, it can also explain why the transmittance of common inorganic gases to these polymers is significantly affected by the content of water vapor in the test gas.
3.2 polymers
The polarity of polymer is the main reason for the strong interaction between water vapor molecules and polymer groups. As mentioned above, the moisture permeability of not all polymers does not have a linear relationship with the humidity difference on both sides of the sample. The test curve (Fig. 1) obtained from the study on the change of pet moisture permeability with the relative humidity difference conducted by Labthink Languang laboratory is basically linear. So what kind of polymer will strongly interact with water vapor and then affect the permeability of materials? Here, polar polymers and hydrophilic polymers will be introduced respectively.
3.2.1 polar polymers
Because hydrogen bonds can be formed between water vapor molecules and polar polymer groups, and hydrogen bonds are the main intermolecular force in polymer materials, the influence of material molecular structure on material permeability can not be ignored. Generally, materials with small molecular polarity or with few polar groups in the molecules have small hydrophilic tendency and low hygroscopic property; Polymer materials with more polar groups such as -coo-, -co-nh- and -oh also have strong water absorption.
According to the effective dipole distance of various groups in the polymer μ, Polymers can be divided into the following four categories according to the size of polarity:
Nonpolar polymer( μ= 0): polyethylene, polypropylene, polybutadiene, polytetrafluoroethylene, etc
Weakly polar polymer( μ ≤ 0.5): polystyrene, natural rubber, etc
Polar polymer( μ> 0.5): PVC, nylon, plexiglass, etc
Strongly polar polymer( μ> 0.7): polyvinyl alcohol, polyester, polyacrylonitrile, phenolic resin, amino plastic, etc
3.2.2 hydrophilic polymer
Although hydrogen bonds can be formed between water vapor molecules and the groups of polar polymers, which will have a certain impact on the barrier properties of polymers, the barrier properties of polymers that are not highly polar are vulnerable to changes in relative humidity. Some literatures point out that the barrier performance of hydrophilic polymers is significantly affected by the concentration of water vapor molecules (relative humidity) (see Figure 2, cellulol is a hydrophilic polymer). For hydrophilic polymers, such as PA6, PVA, EVOH, etc., if the water in the environment diffuses into the material, it is equivalent to adding a certain amount of plasticizer to the material, which will not only increase the free volume in the packaging material, but also make it easy to rearrange some moving units of the polymer, provide more opportunities for the instantaneous gap through which the permeable molecules diffuse, and increase the permeability coefficient of the gas. As mentioned above, generally, polymers with strong polarity also have strong water absorption, and hydrophilic polymers are mainly polar polymers.
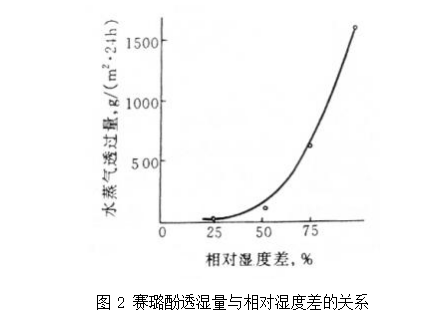
For polymers with weak hydrophilicity and complete hydrophobicity (such as polyolefins and some polyesters), due to their low solubility and diffusivity, the permeation process is similar to that of common inorganic gases. Figure 3 (excerpted from 'plastic testing technology') shows the relationship curve between moisture permeability and relative humidity difference of four hydrophobic polymers, with a good linear relationship, including some polar polymers, such as PVC.
4. Summary
Due to the strong interaction between water vapor molecules and hydrophilic polymers, the moisture permeability of polymers changes nonlinearly with the relative humidity difference on both sides. Moreover, water vapor swells the hydrophilic polymer and changes its structure. Therefore, the penetration of common inorganic gases into the hydrophilic polymer is also affected by humidity. Therefore, when discussing the moisture permeability of polymers, we must clarify the relative humidity difference, temperature and other conditions of the test; When testing the permeability of hydrophilic materials, the humidity in the environment or in the test gas should also be noted as one of the conditions for data comparison.
Hydrophilic materials are rarely used alone. They are mostly used as a layer of multilayer films and protected by other hydrophobic materials to avoid affecting the performance due to moisture absorption. Labthink Languang laboratory also conducted a small number of tests with other films (pure films) during this experimental study, proving that the moisture permeability of hydrophobic materials is approximately linear with the relative humidity difference on both sides of the sample. Since there are few pure NY, EVOH and other materials susceptible to humidity stored in the laboratory, which are not enough to carry out a large number of tests, the test data of hydrophilic materials cannot be provided. Here, film raw material manufacturers are welcome to cooperate with Labthink Languang laboratory to facilitate the interoperability of packaging materials with better barrier and applicability.